Oncocyte Corp. (OCX) management is focused on two things, expansion and execution. With several upcoming events and milestones on the horizon, building significant shareholder value is the primary goal. These milestones include driving awareness and sales uptake for DetermaRx™ in the U.S. facilitated by the recently finalized reimbursement under CMS, leveraging the sizeable global opportunity for DetermaRx™ through distribution and licensing agreements, driving market share with new product extensions and mutation testing with DetermaTx™, expanding the use of DetermaIO™ in pharmaceutical research and clinical trials for immune-therapies. Importantly, management has a solid track record and a history of creating value, even in the most challenging situations.
The Cancer Care Continuum
The diagnosis of cancer is a traumatic and life-altering event. Fortunately, statistics from the National Cancer Institute show that survival rates have steadily increased over the past several decades. This has mostly been due to early detection and advancements in modern medicine. However, depending on the type and stage of the cancer diagnosis, questions remain about the ideal path along the cancer care treatment continuum.
Specifically, within lung cancer, patients and physicians face a problematic question following surgical removal of early-stage (Stage I/IIA) tumors. Despite undergoing intended “curative” surgery, 30-55% of patients with early-stage lung cancer have a recurrence, and unfortunately, very few of these patients with recurrence achieve long-term survival. This is likely because, at the time of surgery, these patients already had micro-metastatic cells that were not removed by performing lung surgery alone, and were undetected in the traditional staging workup of the patient’s tumor.
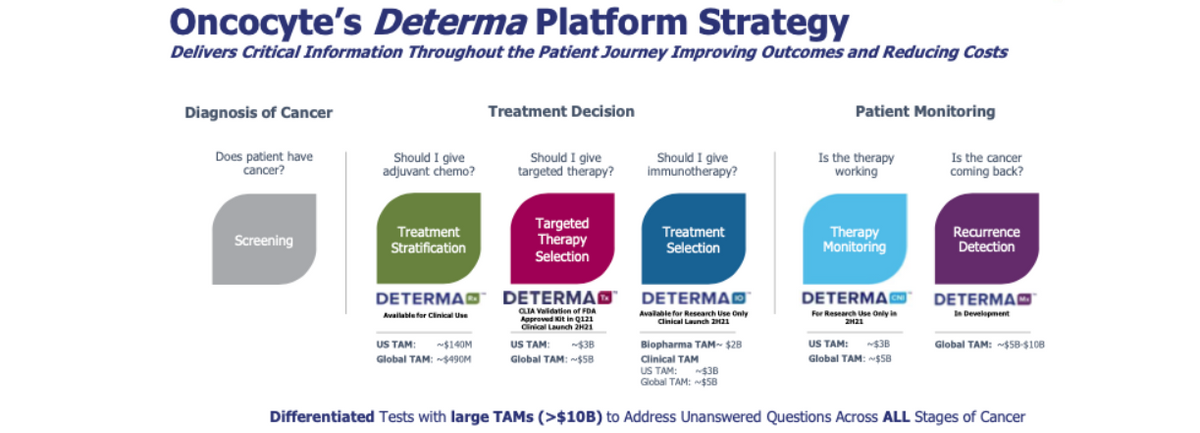
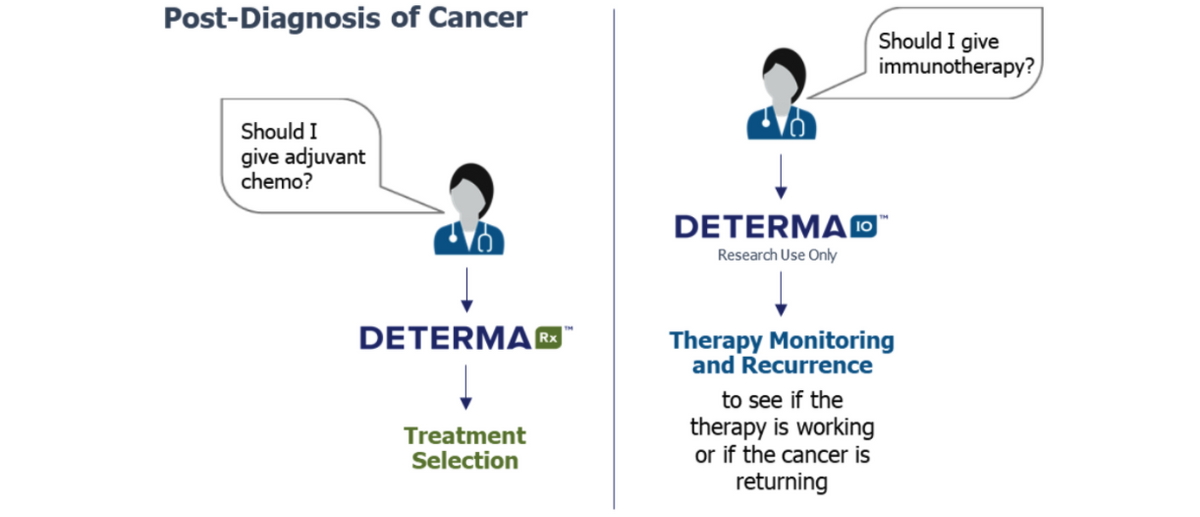
DetermaRx is the first and only predictive test for identifying patients with Stage I/IIA non-squamous NSCLC who are at high-risk for recurrence following surgery and are likely responsive to adjuvant chemotherapy. There are an estimated 40,000 surgically resected Stage I/IIA non-squamous NSCLC patients in the U.S. each year. Including regions outside the U.S., Oncocyte estimates there are 350,000 patients potentially eligible for the test on an annual basis.
Until DetermaRx, there was no reliable way to molecular stage an NSCLC tumor to identify high-risk patients and treat them early with chemotherapy to help avoid recurrence. DetermaRx is a molecular test that measures the gene expression signature of a patient’s tumor by analyzing 14 different genes via quantitative polymerase chain reaction (PCR) to classify patients at high or low risk of recurrence.
Adjuvant chemotherapy is the use of chemotherapeutic agents to prevent the recurrence of cancer in patient’s post-resection of their tumor. After surgery, physicians may recommend platinum-based chemotherapy for intermediate and high-risk patients, while suggesting a “watch-and-see” approach with low-risk patients.
Chemotherapy has side effects, such as the potential for serious adverse events and neurotoxicity, as well as the potential to trigger more aggressive tumors, so the decision to prescribe adjuvant chemotherapy is not taken lightly. Currently, the National Comprehensive Cancer Network (NCCN) guidelines suggest clinicopathologic characteristics to identify high-risk patients for adjuvant intervention. However, none of these characteristics have been validated as predictive biomarkers in large peer-reviewed studies and, as such, have not been broadly adopted by physicians to guide their decision.
Results from Oncocyte’s landmark DetermaRx clinical studies published in leading medical journals such as JAMA and Lancet show that the test is not only more accurate than conventional clinicopathologic approaches in identifying patients at high-risk for recurrence, but that the clinical outcomes can be vastly improved when these high-risk patients are given timely chemotherapy.
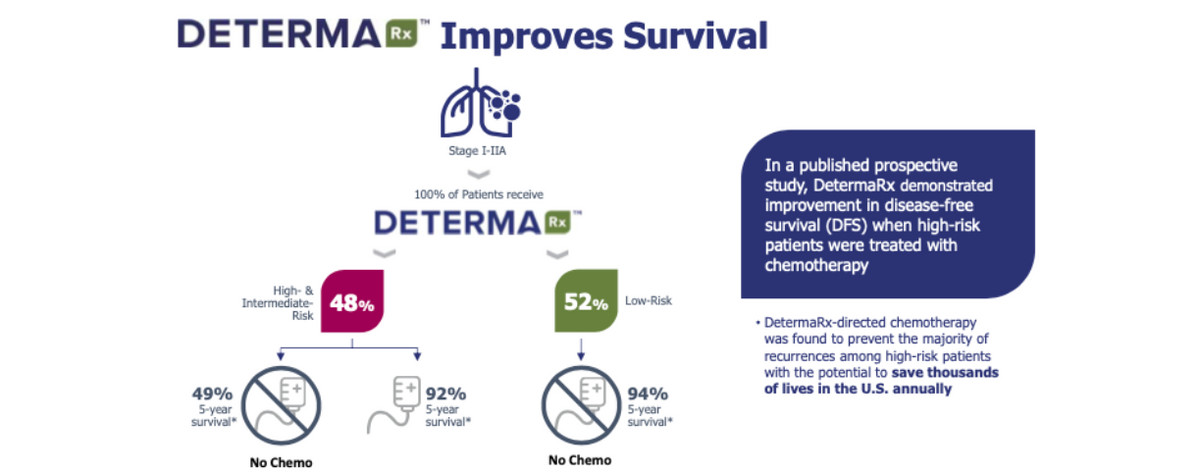
Results published in Clin. Lung Cancer (Woodard GA et al., 2018) showed that 48% of DetermaRx high-risk patients were classified as low-risk by NCCN criteria. Moreover, these patients had a significantly improved DFS compared to high-risk NCCN patients.
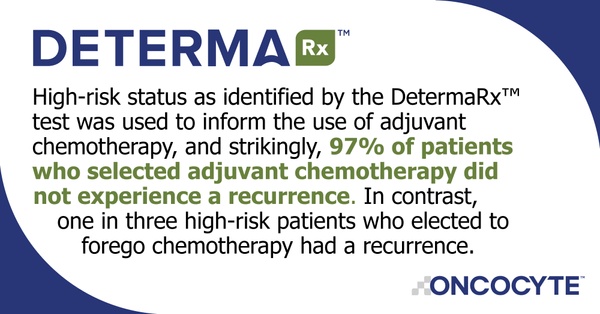
New data from a prospective clinical study were just recently presented by Gavitt Woodard, MD, Assistant Professor, Yale School of Medicine, at the ISALC 2020 North American Conference on Lung Cancer in October 2020. These data show that when DetermaRx was used to inform treatment options, 94% of high-risk patients who selected adjuvant chemotherapy were cancer-free after five years of follow-up. In contrast, one in three high-risk patients who elected to forego chemotherapy had a recurrence. Importantly, DetermaRx identified high-risk patients who responded to adjuvant chemotherapy, independent of EGFR status. Conversely, no DetermaRx-identified low-risk patients were treated with chemotherapy, and only 5% of them reported a cancer recurrence, suggesting that this test may also inform the choice to avoid potentially unnecessary but toxic chemotherapy. These were truly outstanding results that meaningfully improved survival for early-stage lung cancer patients.
Focused On DetermaRx™ Roll-Out
Oncocyte launched DetermaRx™ earlier in the year through an early access program. The product was promoted by a highly accomplished and focused sales force of six representatives in the U.S. for the majority of 2020. These sales professionals have more than ten years of experience selling molecular tests at some of the world’s leading Molecular Diagnostic companies. The company recently expanded the sales force and expects to enter 2021 with ten full-time sales reps. Using an industry, “million-dollars per representative” benchmark, it is easy for shareholders to get a sense of the revenue trajectory expected in the coming quarters.
The trajectory will no doubt be aided by the recently obtained final Medicare reimbursement in September 2020, a significant milestone that secures payment for ~70% of the eligible patients.
There are clear signs that finalized reimbursement is having a material impact on uptake. In the first year of launch the test has been adopted by over 93 hospitals are DetermaRx available, including multiple leading centers in the National Comprehensive Cancer Network (NCCN) and National Cancer Institute (NCI) network. Oncocyte has been able to increase adoption at major healthcare systems including HCA Healthcare, Florida Cancer Specialists (FCS), Scripps Health, Providence Cancer Institute, Banner MD Anderson, St Jude Specialty, Sutter Health, Jupiter Medical Center, and Swedish Cancer Institute, among others. This is comparable to the adoption achieved in the first year by the standard of care tests for the same decision point in breast cancer.

Reorder rates exceed 60%, about twice what comparable tests for breast cancer typically run, and KOL interest in the product is high, as evidenced by the over 1,800 individuals that attended the company’s most recent virtual training webinar. If things continue to go well, the company will look to expand the size of the sales force in the coming quarters.
Importantly, Oncocyte has been active in expanding the market opportunity for DetermaRx by signing licensing agreements, including a deal inked with CORE Diagnostics for India, the Middle East, and Africa in April 2020, a deal with ProGenetics Ltd for Israel in July 2020, and most recently, a deal with Burning Rock Biotech Limited for China in December 2020. The deal with Burning Rock is particularly exciting because Burning Rock is one of the fastest-growing and largest companies in China’s next-generation sequencing (NGS) based cancer therapy selection market. Burning Rock will pay up to $6M for specified deliverables. Outside of those regions, distribution exists in Israel, Latin America, and the company has plans to enter the “EU Big 5” in the coming quarters. Oncocyte estimates that the total addressable market opportunity is approximately $500 million, subject to pricing and adoption rates.
Expanding The Opportunity with DetermaIO™ & DetermaTx™
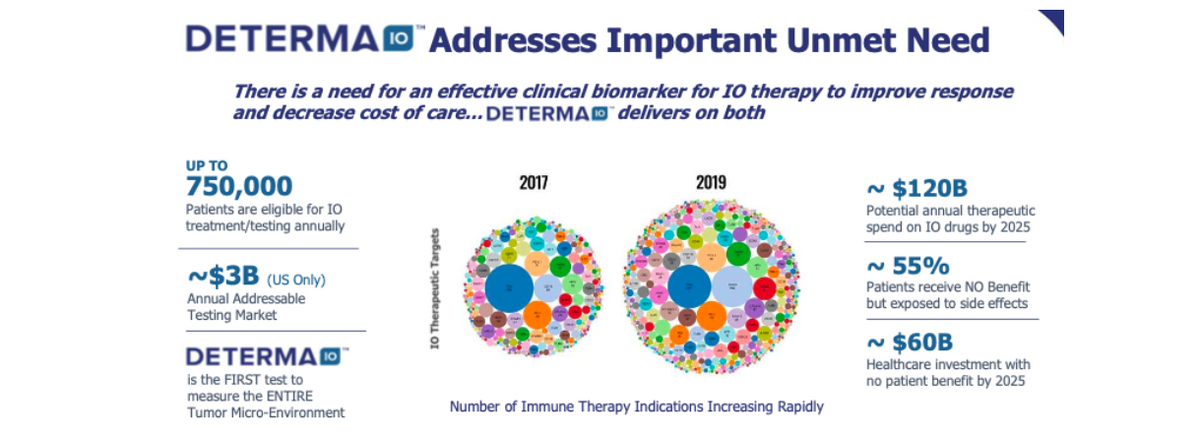
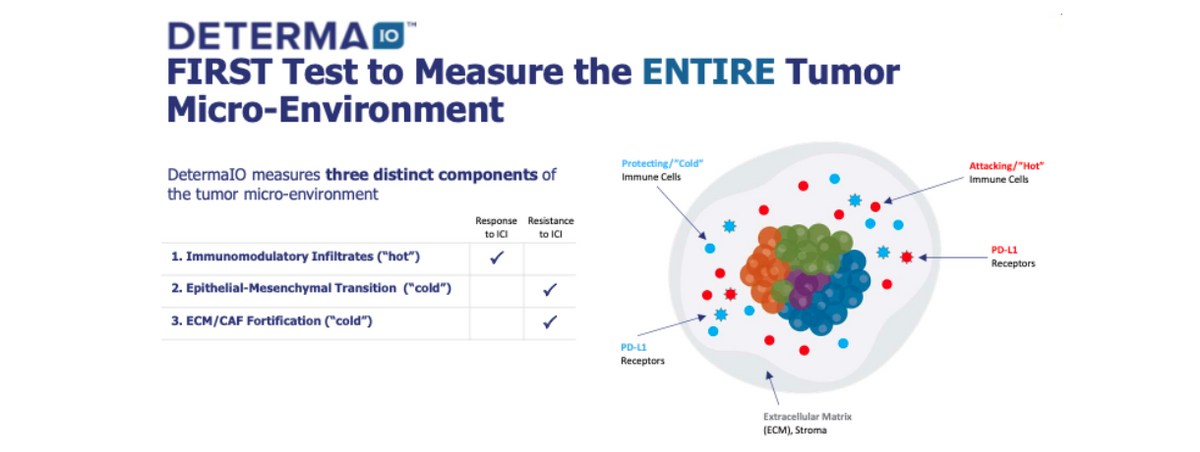
In February 2020, Oncocyte Corp. acquired privately-held Insight Genetics, a Nashville based company that developed a potentially transformative molecular diagnostic product in DetermaIO™. DetermaIO is a CLIA validated test currently available for research use in clinical trials. The test uses real-time PCR to measure expression of 27 genes and a proprietary immune-oncology (IO) algorithm with demonstrated predictive capabilities for patient response to immune checkpoint inhibitor (ICI) therapies in non-small cell lung cancer (NSCLC) and triple-negative breast cancer (TNBC). The biology behind the test, which assesses the entire tumor microenvironment, may identify resistance mechanisms that may be overcome by second generation therapies. ICI’s, such as that anti-PD-1/PD-L1 monoclonal antibodies, Merck’s Keytruda® (pembrolizumab) and Bristol-Myers Opdivo® (nivolumab), have become the new standard of care for certain types of solid tumors, including NSCLC. They are among the largest and fastest-growing segments of cancer therapeutics. The anti-PD-1/PD-L1 class is expected to generate sales in excess of $47 billion in 2024, according to EvaluatePharma.
The DetermaIO test differentiates itself from the current leading immunotherapy diagnostic tests such as PD-L1 and Tumor mutational burden (TMB) by assessing the tumor microenvironment (TME), to determine if the immune microenvironment is active (“hot”) or quiescent (“cold”). Recent data presented by researchers at the MD Anderson Cancer Center at the American Society for Clinical Oncology (ASCO) meeting in May 2020 suggested DetermaIO may be superior to two currently marketed predictive companion diagnostic tests for immunotherapy selection, PD-L1 IHC staining and tumor mutational burden (TMB) (Iwase T et al.).
Tissue insufficiency is a major issue for molecular tests given the multitude of tests that must be performed on diagnostic biopsy samples. In lung cancer 15-30% of patients may not have enough tissue for testing. DetermaIO requires very little tissue and can be successfully performed on most core biopsies. At the Association for Molecular Pathology (AMP) annual conference in November 2020, new data from DetermaIO demonstrate that the test is reproducible on tissue inputs from as little as 2mm2 of tissue. This is the amount contained on a single slide! This tissue requirement is as much as ten-fold lower than required for some marketed NGS gene panel tests. For example, PD-L1 testing requires 3-5 slides while tumor mutational burden (TMB) requires as many as ten slides for testing, oftentimes exhausting the tumor tissue available.
This type of test has excellent utility for biopharmaceutical companies working on targeted immunotherapies looking to add incremental value on top of standard-of-care anti-PD-1 treatment. For example, there are over 3,000 active clinical studies in the U.S. alone with immunotherapies. Existing patient selection criteria fall short with patient stratification for anti-PD-1, or ideal use of combination targeted therapy on top of anti-PD-1, such as IL-12, anti-VEGF, TIGITs, DKK1, CD47, or other targeted agents. This type of test is also very attractive to pharmaceutical companies looking to expand indications for PD-1 and PD-L1 inhibitors to earlier stages of cancer and new indications, and to identify new patient populations not identified by the standard of care biomarkers such as PD-L1 and TMB.
On the other hand, because this test can also identify non-responders to treatment, this type of analysis is particularly attractive to commercial payers and health systems that might spend upwards of $15,000 per month for Keytruda® treatment. Oncocyte is beginning to see customer adoption of DetermaIO here in late 2020. For example, in October, the company announced a strategic collaboration with Fondazione Michelangelo, a leading non-profit cancer centers research foundation in Italy, whereby Fondazione will evaluate DetermaIO as a biomarker for response in the NeoTRIPaPDL1 (NCT02620280) clinical trial for triple-negative breast cancer.
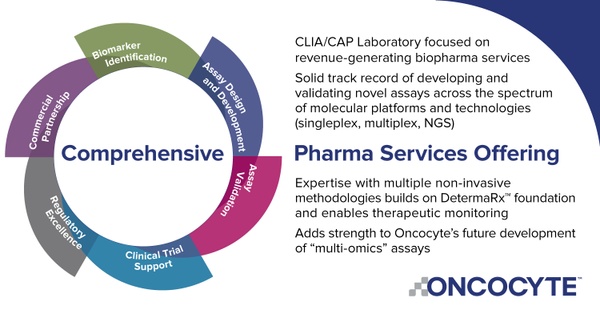
Beyond just acquiring DetermaIO, the acquisition of Insight Genetics also expands Oncocyte’s clinical operations capabilities by providing the company with a CLIA certified and CAP-accredited lab with the certifications required to submit data to regulatory agencies around the world. Oncocyte believes the acquisition is immediately accretive to its pharma services business.
Having the ability to partner with pharma companies and assist in their global regulatory approvals will help the company grow significant new opportunities for pharma services in the expanding immunotherapy and companion diagnostic markets.
For example, in September 2020, Oncocyte announced a strategic collaboration with Guardian Research Network (GRN) to create a comprehensive solution for pharma clients from patient recruitment to regulatory approvals. Oncocyte and GRN aim to leverage their joint capabilities by bringing together GRN’s clinical trial enrollment and data science technology with Oncocyte’s proprietary molecular tests, as well as Oncocyte’s pharma and companion diagnostic development services.
DetermaIO is currently approved for research use, allowing Oncocyte to take a two-step approach to value creation. Firstly, the company is looking to partner with biopharma companies and cancer research centers, like GRN and Fondazione, looking to incorporate DetermaIO into their clinical programs, for which Oncocyte will sell DetermaIO. Subsequently, Oncocyte can then use the clinical data generated from these studies to file applications for full commercial approval in the coming years. Once fully approved for commercial use, Oncocyte believes that DetermaIO represents a potential $3 billion total addressable market opportunity.
In the meantime, as noted above, the company can generate revenues from research collaborations and work to expand its Pharma Services business, which provides precision medicine services to pharmaceutical companies looking to develop companion diagnostics. Oncocyte is one of the only companies in the world that can offer clinical support operations that include concept design, clinical trial support, and commercial lab services to pharmaceutical clients. And importantly, Oncocyte is set on expanding its products and services. The company plans on providing DetermaTx, a targeted genomic test to help physicians and potential pharmaceutical partners identify patients for the right therapy based upon their molecular profile. This allows Oncocyte to complete the continuum by offering tests that will give physicians all the data needed to make an informed treatment decision for the patient’s another potential $5 billion global market and an excellent complement to DetermaIO, immuno-therapy diagnostic. Among DetermaRx, DetermaIO, and now DetermaTx, Oncocyte is helping craft the treatment paradigm within the global $10 billion cancer diagnostic market.
A Move Into Monitoring
Not all patients are cured with surgery or respond to treatment, After a cancer diagnosis and treatment decision, patients require monitoring to identify the presence of disease (MRD) or progression on therapy (therapy monitoring). These tests must be blood based, cost effective for repeat use and accurate in predicting disease. This is another potential multi-billion dollar market opportunity and Oncycyte is positioning itself to be a player just like in diagnostics. In October 2020, the company entered into an agreement with Chronix Biomedical whereby Oncocyte will license the TheraSure™-CNI MONITOR clinical assay. Rebranded DetermaCNI™, this is a completely blood-based liquid biopsy test that uses copy number instability (CNI) to sensitively quantify the cell-free DNA from the primary solid tumor in a patient’s blood. Measuring changes in CNI scores over cancer treatment can help assess cancer patients’ response to therapy in real-time. It’s an excellent complement to Oncocyte’s DetermaRx, DetermaIO, and DetermaTx products and creates a “one-stop-shop” where Oncocyte can provide services throughout the entire continuum, from diagnosis to monitoring. Blood-based therapeutic monitoring and recurrence detection products, through yet another product, to be called DetermaMx, should be available for research use in the second half of 2021 and 2022, respectively. Importantly, as part of the collaboration with Chronix, Chronix will use its proven laboratory network in Germany to accelerate the EU market commercial launch of Oncocyte’s diagnostic products in 2021.
Expansion & Execution
Over the next several months, investors should be on the lookout for milestones that should create significant value at Oncocyte. With DetermaRx, management is focused on driving volume growth and expanding uptake into the private market based on the positive CMS coverage determination earlier in the year. Management has already signed two deals for DetermaRx outside the U.S. and is focused on expansion in China with Burning Rock and into Europe as the next primary market for the product. With DetermaIO, Oncocyte is squarely focused on engaging with biopharma partners for research use of the product, building the evidence to secure Medicare reimbursement to drive clinical adoption, and expanding the pharma services it can offer to develop new and customized assays. The same strategy exists for DetermaTx.
Oncocyte is uniquely positioned with a growing suite of proprietary tests that could profoundly change how cancer is managed and how immunotherapy is utilized across all solid tumors, and are at the inflection point of commercialization in both indications representing a global TAM of over $10 billion.
Oncocyte is a precision diagnostics and monitoring company with the mission to improve patient outcomes by providing clear insights that inform critical decisions in the diagnosis, treatment, and monitoring of cancer. The Company, through its proprietary tests and pharmaceutical services business, aims to help save lives by accelerating the diagnosis of cancer and advancing cancer care. The Company’s tests are designed to help provide clarity and confidence to physicians and their patients at every stage. DetermaRx™ identifies early-stage lung cancer patients who are at high risk for cancer recurrence and who may benefit from adjuvant chemotherapy. DetermaIO™, a gene expression test currently used as a research-use only tool, assesses the tumor microenvironment to predict response to immunotherapies. The Company’s pipeline of tests in development also includes DetermaTx™, which will assess mutational status of a tumor, blood-based monitoring test DetermaCNI™, and long-term recurrence monitoring test DetermaMx™. In addition, Oncocyte’s pharmaceutical services provide companies that are developing new cancer treatments a full suite of molecular testing services to support the drug development process. DetermaRx™, DetermaIO™, DetermaTx™, DetermaCNI™ and DetermaMx™ are trademarks of Oncocyte Corporation.
Oncocyte Forward-Looking Statements
Oncocyte cautions you that this press release contains forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates,” “may,” and similar expressions) are forward-looking statements. These statements include those pertaining to the DetermaIO and DetermaCNI tests, including the potential of DetermaIO to fulfill the unmet need for optimizing the use of immunotherapy for more than 1 million patients who are eligible for this treatment each year, the expectation that DetermaIO will launch clinically as part of an early access program later this year and the potential of DetermaIO and DetermaCNI to enable oncologists to manage patients longitudinally during the course of their cancer treatment. Forward-looking statements involve risks and uncertainties, including, without limitation, the potential impact of COVID-19 on Oncocyte or its subsidiaries’ financial and operational results, risks inherent in the development and/or commercialization of diagnostic tests or products, uncertainty in the results of clinical trials or regulatory approvals, the capacity of Oncocyte’s third-party supplied blood sample analytic system to provide consistent and precise analytic results on a commercial scale, potential interruptions to supply chains, the need and ability to obtain future capital, maintenance of intellectual property rights in all applicable jurisdictions, and the need to obtain third party reimbursement for patients’ use of any diagnostic tests Oncocyte or its subsidiaries commercialize, and risks inherent in strategic transactions such as the potential failure to realize anticipated benefits, legal, regulatory or political changes in the applicable jurisdictions, accounting and quality controls, potential greater than estimated allocations of resources to develop and commercialize technologies, or potential failure to maintain any laboratory accreditation or certification. Actual results may differ materially from the results anticipated in these forward-looking statements and accordingly such statements should be evaluated together with the many uncertainties that affect the business of Oncocyte, particularly those mentioned in the “Risk Factors” and other cautionary statements found in Oncocyte’s Securities and Exchange Commission filings, which are available from the SEC’s website. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. Oncocyte undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
For more information, please visit https://oncocyte.com/ or follow us on Twitter at @OncocyteCorp, Facebook, and LinkedIn.